DUOTex® Introduction

The ideal surgical outcome of a dental implant is stability and a sustained connection to surrounding bone. Achieving this outcome relies on a number of factors, not the least of which is the formation of a tight, sealed bond between implant and bone. Implant design, and specifically the surface properties of the implant, have a profound impact on osseointegration, and several clinical studies support this {1,2}.
Various cell culture experiments have demonstrated that the modification of surface architecture has a positive impact on cell proliferation and the cytokine and growth factor production of osteoblast cells {3,4}. Animal studies support these results, by showing a high correlation between increased surface roughness and the level of bone-implant contact {5}. Finally, clinical trials show the use of implants with a rough surface accelerate the apposition of osteoblasts on the implant surface {6,7,8}.
Certain aspects of surface architecture, such as depth, extension and average roughness of the pores, are of great importance in surface treatments as they not only influence the cellular orientation, but also contribute to improving force transmission. A rough surface architecture also raises the concentration of PGE2, BMP-2 and TFG-ß on the surface of the implant, whereby the pores or depressions in the surface form a solid matrix for the absorption and retention of osteogenic proteins. Surface topology also exerts a major influence on the quality and kinetics of osseointegration {9}, and hydrophilicity promotes a reduction in surface tension, improving cellular adhesion.
Implant surface technology may be achieved via additive or subtractive processes. Studies show that subtractive processes achieved with blasting and subsequent acid etching result in a microtexture that supports osseointegration {10,11,12}. A comparative study of various treated titanium surfaces showed a blasted/etched surface had more positive outcomes in relation to bone contact than in surfaces modified with additive processes such as titanium plasma spray [TPS] or otherwise {13}. Davies & Dziedzic showed greater bone apposition with blasted/etched surfaces than with smooth, machined surfaces, ascribing the blasted/etched surfaces an osteoconductive effect as a result of the microtexture {14}.
In short, an effective implant surface accomplishes multiple objectives:
- An anchor surface that supports a three-dimensional biological matrix for osteogenic cells{15}
- High resistance to tension and shear forces
- Sustained connection with surrounding bone
- Good biocompatibility
- Successful cell proliferation and osseointegration {16,17}
DUOTex® Introduction
I. Structure and Surface
DUOTex® is a macro- and microstructured surface generated via blasting with hydroxyapatite [HA] powder and subsequent acid etching. The DUOTex® surface is comprised of titanium and titanium oxide, according to ISO 5832-2, and is biocompatible. From a chemical standpoint, the surface is not modified and shows no contamination from the HA powder.
II. Physical Properties
A. Roughness
The average surface roughness of the DUOTex®-treated implant is determined with the tactile stylus method according to DIN EN ISO 4287 with the Hommel Tester T 8000. Average roughness of the DUOTex® surface is Ra = 1.1 ± 0.5 μm (Fig. 1).
B. Hydrophilicity
The contact angle of a liquid on a material informs the extent of the contact the liquid has with said material. The average water contact angle of the DUOTex® surface is 100°, or slightly hydrophilic. This assists in reducing surface tension, thus improving cellular adhesion.
C. Shelf-Life
DUOTex® surfacing is free of organically or inorganically unstable compounds, and has a shelf life of at least 5 years at in its original packaging at normal storage temperatures.
III. Biological Properties
A. In Vitro Cultivation of Cells
Cultivation of osteoblasts on DUOTex® surfacing showed positive cell proliferation and differentiation, as well as typical cell structure.
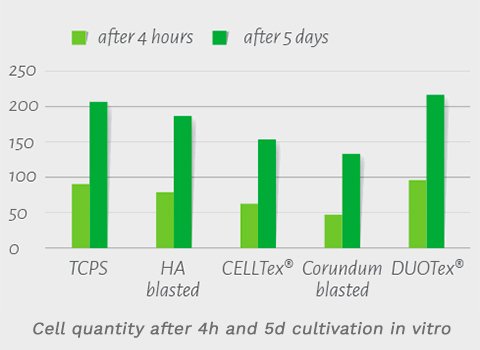
- In a comparative study of cell proliferation on a range of surfaces, human stem cells were cocultivated on titanium test specimens with various surface modifications. TCPS as a conventional surface of the cell culture was the control. Figure 3a-1 shows cell quantities after 4 hours and 5 days of cultivation, and the significant increase in cell quantity on the DUOTex® surface is clear.
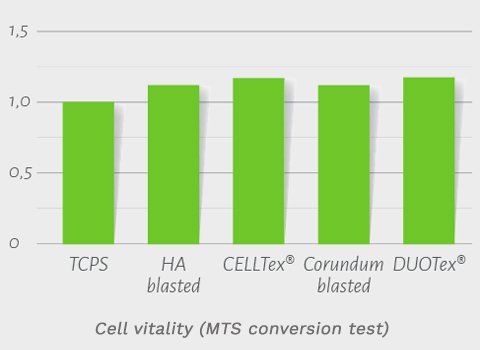
- Cell vitality was studied via the MTS conversion test and showed nearly 100% vitality on the DUOTex® surface, indicating excellent biocompatibility (Fig. 3a-2).
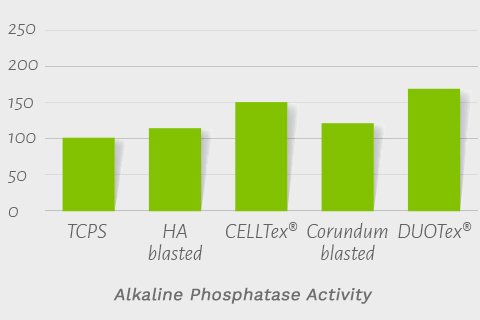
- The alkaline phosphatase (ALP) activity of the cultivated cells refers to a glycoprotein found on the cell surface of the osteoblasts, and is involved in the calcification of bone matrix {18}. Figure 3a-3 shows increased phosphatase activity on the DUOTex® surface as compared to the control TCPS surface, indicating increased osteogenic differentiation.
B. Cultivation of Differentiated Cell Types on DUOTex®
To further investigate biocompatibility, an osteosarcoma cell line (MG63) and human dermal endothelial cells (HDMEC) were cultivated for several days on the DUOTex® surface. The morphology of the various cells were then analyzed and evaluated with vital nuclear staining and SEM images. In nuclear staining, the cell nuclei are stained blue and the cytoplasm of the cells green. Non-vital cells show a clear red coloration.
- Figure 3b-1 shows the vital and nuclear staining of the osteosarcoma cell line MG63 on the DUOTex® surface. The cells demonstrate typical osteoblast morphology, they are vital and form a semi-confluent to confluent cell lawn on the tested surface.
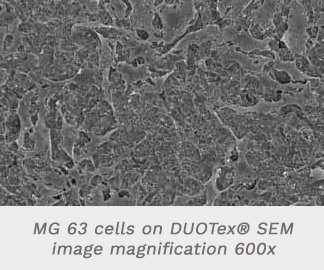
- The SEM image in Figure 3b-2 shows an almost confluent cell lawn on the DUOTex® surface, whereby the cells show morphology typical for osteoblasts (Fig. 3b-3).
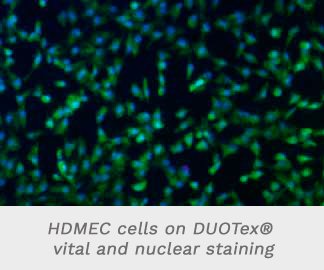
- Figure 4a shows the vital and nuclear staining of the HDMEC cells on the DUOTex® surface, again demonstrating confluent cell lawn with typical cell morphology and a high vitality rate (Fig. 4b, 4c). cellular adhesion.




DUOTex® Process Flow

DUOTex® Testing
I. Chemical Testing
Due to exposure to acids in the production process, the concentration of acid ions on the DUOTex ® surface were tested via residue analysis (NORDUM Institute für Umwelt und Analytik GmbH & Co. KG). The results showed residual acid ion values below the limit of determination, indicating no detectable levels of residual acid ions were found on the implant surface {19}. An EDX analysis performed on the DUOTex®-treated implants showed pure titanium with a non-chemically modified surface and no impurities {20}.
II. Physical Testing
In addition to measurements for surface roughness, surface structure is also measured. SEM images, or scanning electron microscopy, with 2000 X magnification are produced and implants are tested for homogeneous surface structure without flaws or irregularities (Fig. 5a-c).


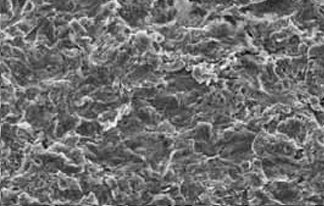
SEM images of a flawless DUOTex® coating (magnification 2000x)
III. Biocompatibility Testing
As the length of contact between DUOTex®-treated implants and biological tissue exceeds 30 days, tests for cytotoxicity were conducted according to applicable standards. The test for cytotoxicity was performed both with untreated implant blanks as well as with DUOTex®-treated implants. The in-vitro cell toxicity test was performed on mouse fibroblast cells in accordance with DIN ISO 10993-5 (DIN EN 30993-5) guidelines. No morphological changes or cytoplasmic damages to the cell were detected, and there was no biological-toxicological damage to the test cells. Accordingly, the DUOTex® surface meets the requirements for biological compatibility in-vitro and is classified as non-toxic within the parameters of DIN ISO 10993-5 {21}.
IV. Testing Summary
| Test Criterion | Result |
| Color | Dark Grey |
| Roughness | Ra = 1.1 ± 0.5 μm |
| Residue Analysis | Acid residues below the limit of detection |
| EDX Analysis | No contamination, no acid residual ions |
| Cytoxicity | Not cytotoxic (in accordance with DIN EN ISO 10993-5) |
| Surface Structure | Uniform structured in the SEM image |
| Durability | 5 years |
DUOTex® Clinical Data
I. Animal Studies
No new animal studies were conducted as the DUOTex® surface treatment was studied extensively in clinical trials and titanium is an established biocompatible material widely used in the field of implantology.
II. Clinical Use
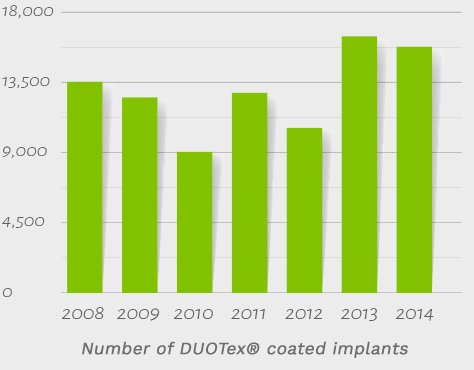
93,000 DUOTex®-treated implants have entered the market between 2008 and 2014, with no negative feedback in that time frame from the end user. The DUOTex® surface has proven itself as an outstanding option in a variety of surgical scenarios, including immediate loading, immediate restorations and delayed restorations with conventional submerged healing.
DUOTex® Summary
In summary, it has been demonstrated that the surface architecture of an implant has a profound and lasting effect on surgical outcome and healing. The unique properties of the subtractive DUOTex® implant surface allow for a biocompatible anchor that supports the proliferation of osteogenic cells, high resistance to tension and shear forces and sustained connection and osseointegration with surrounding bone.
